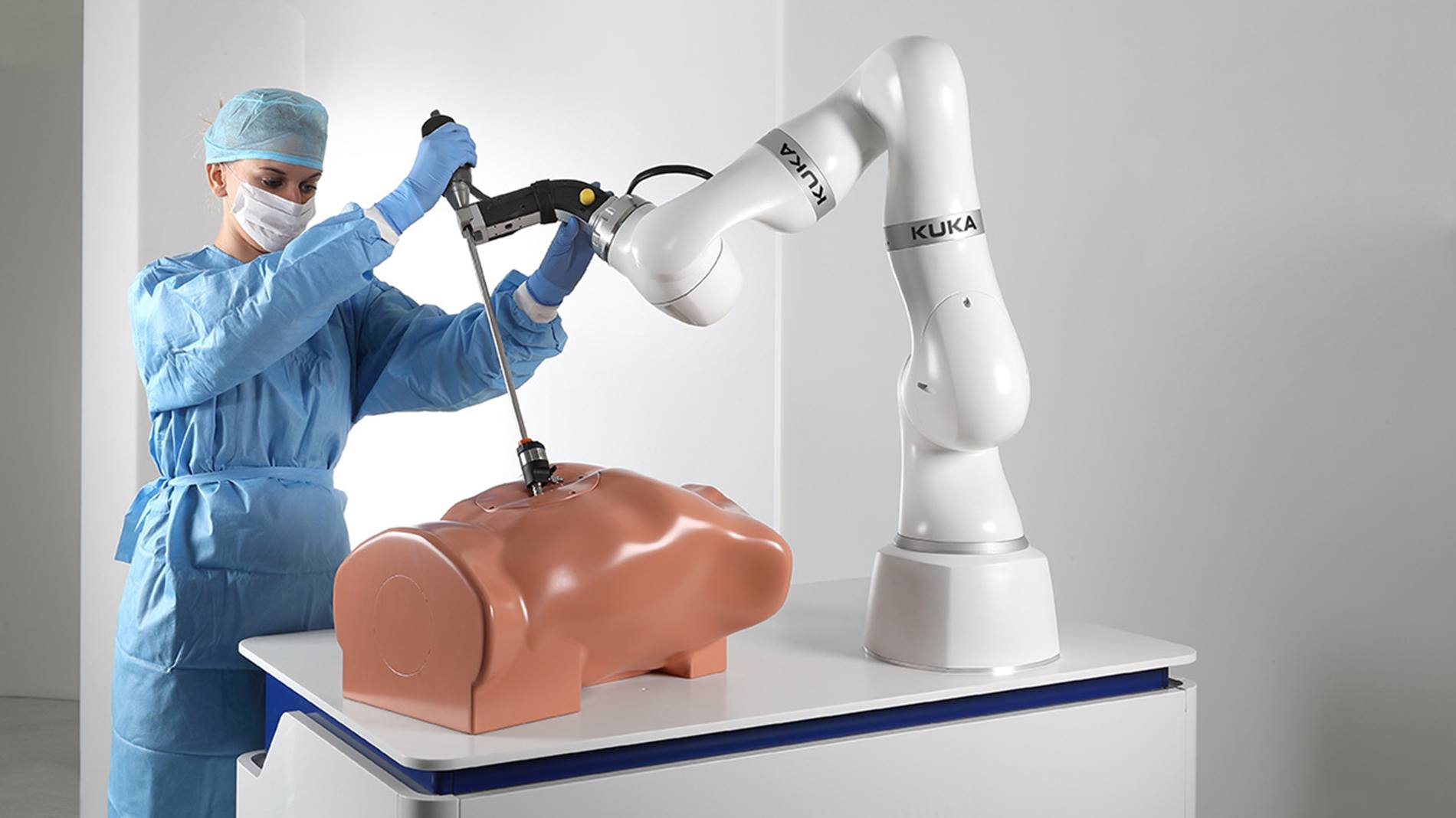
LBR Med meets the regulatory requirements of IEC 60601-1
KUKA has made extensive adaptations to enable operation of the innovative lightweight robot under the strict requirements of medical treatment and interventions. It has biocompatible and corrosion-resistant surfaces. Internally routed connections offer a further point of compliance with the hygienic standards in doctors’ offices, clinics and operating rooms. KUKA also guarantees inspection of the LBR Med through the IECEE CB scheme according to standard IEC 60601-1. With the CB Certificate and CB Report from KUKA, complex testing is reduced and certification procedures are made significantly easier. KUKA already takes care of this in the factory.